New strategy facilitates asymmetric multicomponent coupling reactions of olefins
Return to ListYifeng Chen's group, a professor at the School of Chemical and Molecular Engineering, East China University of Science and Technology, and the Feringa Nobel Prize Scientist Joint Research Center, has made new progress in the asymmetric multi-component coupling reaction of olefins. The related research was published online in the Journal of the American Chemical Society.
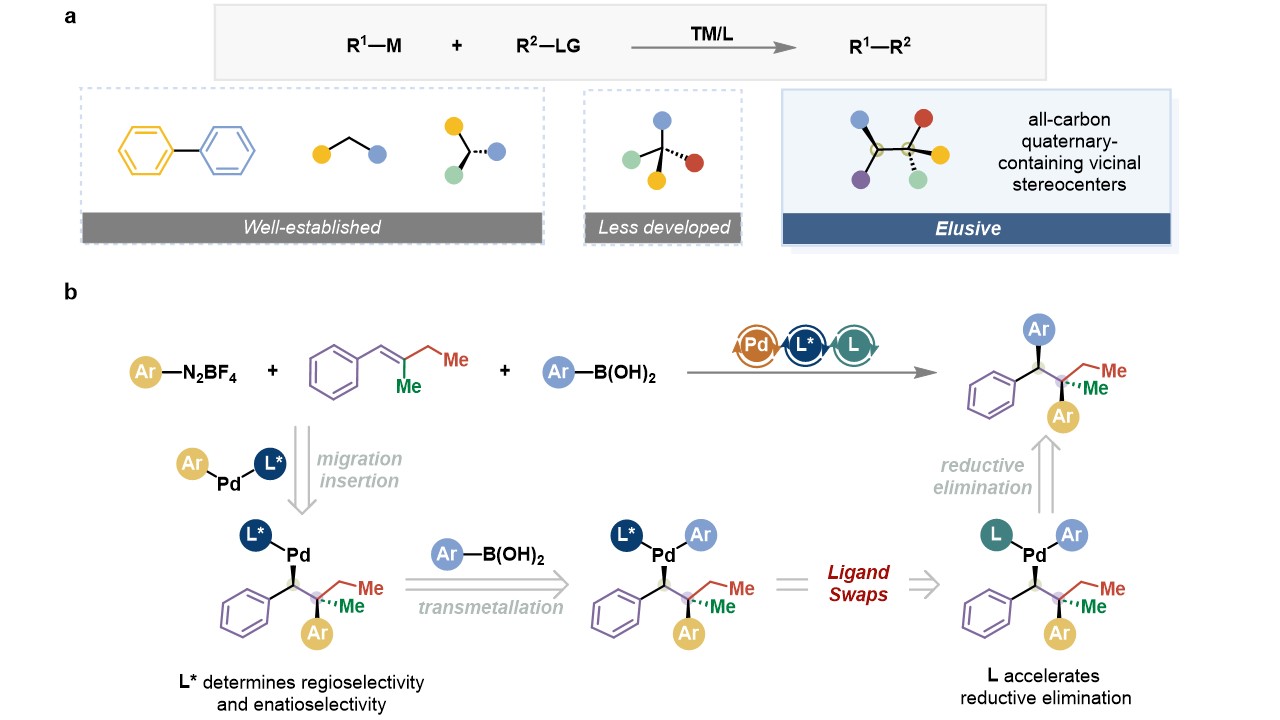
In recent years, transition metal-catalyzed asymmetric coupling reactions have become one of the most direct and effective strategies for constructing various types of Csp3 stereocenters and have been widely used in the synthesis of natural products and drug molecules. However, the construction of all-carbon quaternary carbon centers using transition metal-catalyzed intermolecular asymmetric coupling reactions still poses great challenges, and the asymmetric coupling reactions of continuous chiral centers containing all-carbon quaternary carbons have rarely been reported.
The group has realized the palladium-catalyzed asymmetric 1,2-bisarylation reaction of multicomponent between molecules of undirected trisubstituted olefins using the strategy of double ligand exchange co-catalysis, and constructed the continuous chiral centers containing quaternary carbons with exclusive regioselectivity and diastereoselectivity as well as high enantioselectivity. In addition, in collaboration with the group of Professor Huang Genping of Tianjin University, the team elucidated the details of the enantioselective regulation of the reaction and the role of the bis-ligand in the reaction by DFT calculations, i.e., the chiral bis-oxazolidine ligand modulates the enantioselectivity during the migratory insertion; the achiral fumarate accelerates the reduction-elimination step, which leads to the improvement of the efficiency of the reaction, and the strategy efficiently avoids the use of the guide group.
Research Brief. Image courtesy of Journal of the American Chemical Society
Related paper information: https://doi.org/10.1021/jacs.4c05480




